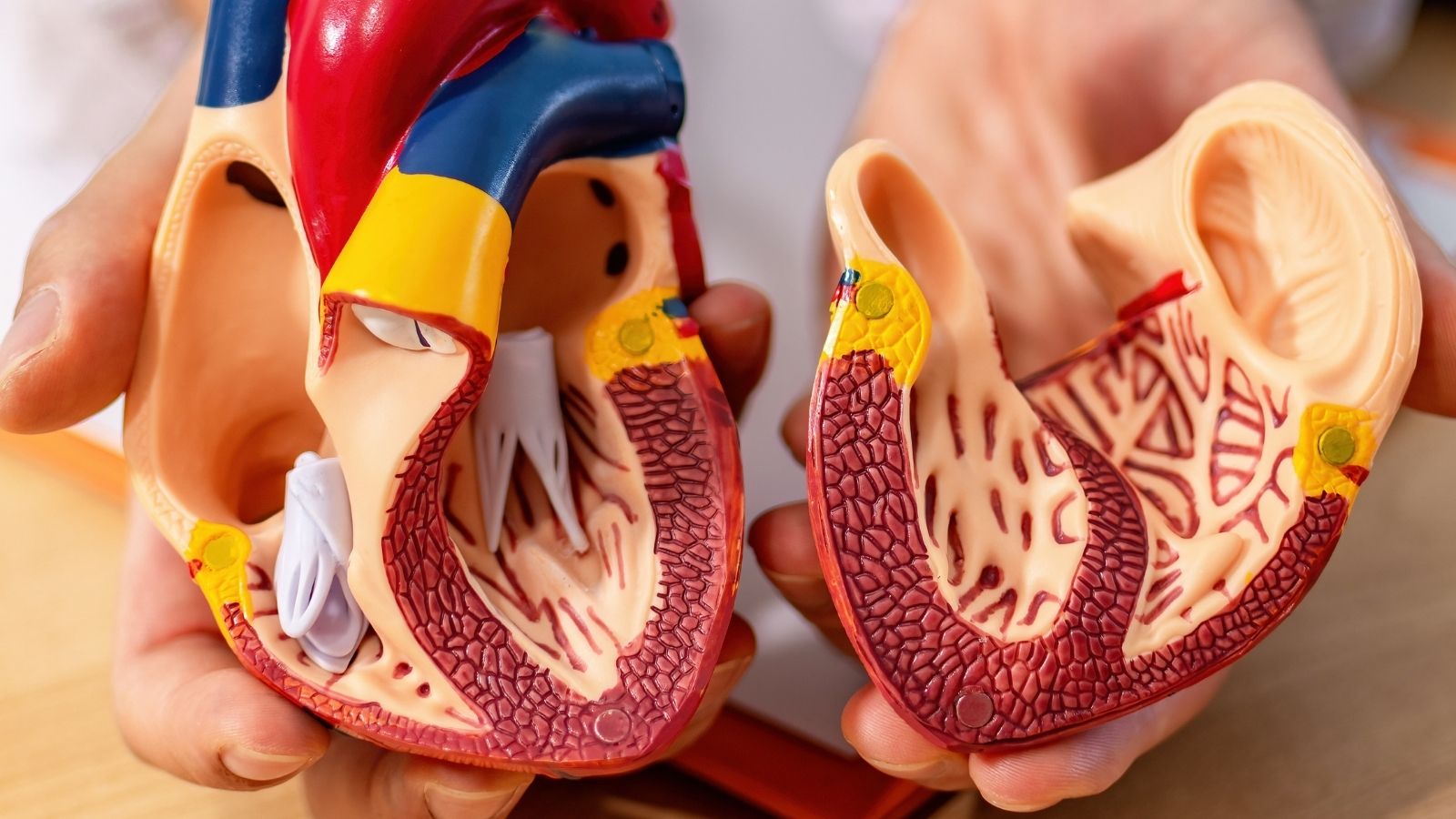
Patent ductus arteriosus (PDA) is a congenital heart defect where the fetal connection between the aorta and pulmonary artery fails to close after birth. This causes abnormal blood flow and overloading of the lungs and heart.
Clinical presentation varies from asymptomatic cases to symptoms such as rapid breathing, poor feeding, and failure to thrive in infants. In severe cases, heart failure may develop if untreated.
Diagnosis is established through echocardiography, which visualizes the persistent ductus and assesses hemodynamic impact. Chest X-ray and ECG may provide supportive findings.
Treatment options include pharmacological closure with NSAIDs in infants, catheter-based interventions, or surgical ligation, depending on patient age and clinical severity.
| Medical Name | Patent Ductus Arteriosus (PDA) |
| Common Symptoms | – Rapid breathing- Difficulty feeding (in infants)- Rapid fatigue- Growth retardation- Heart murmur- Frequent respiratory infections |
| Causes | – The ductus arteriosus, which should normally close after birth, remains open |
| Risk Factors | – Prematurity (preterm birth)- Maternal infections (such as rubella during pregnancy)- Family history of congenital heart disease |
| Complications | – Heart failure – High pressure in the pulmonary vessels (pulmonary hypertension) – Risk of endocarditis – Developmental delay |
| Diagnostic Methods | – Physical examination (hearing a murmur in the heart)- Echocardiography- Chest X-ray- ECG |
| Treatment Methods | – Drug treatment (prostaglandin inhibitors in premature babies)- Catheter closure- Surgical closure (if necessary) |
| Prevention Methods | – Regular follow-up of risky pregnancies – Prevention of maternal infections – Regular postnatal infant checks |
What is Patent Ductus Arteriosus (PDA)?
Patent Ductus Arteriosus, or PDA for short, is a condition in which a vascular connection between the two large arteries coming out of the heart, which should normally close immediately after birth, remains open. Instead of thinking of this condition as a heart “defect” or “hole”, it is more accurate to see it as a normal structure, which is vital for life in the womb, forgetting to close after birth.
To understand this better, let us take a brief look at the circulatory system of a baby in the womb. In the womb, the baby’s lungs are not yet filled with air and do not have the task of oxygenating the blood. The baby receives the oxygen and nutrients it needs from its mother’s blood through the placenta. In this unique arrangement, it is much more efficient for the blood circulation to be distributed directly throughout the body without passing through the non-functioning lungs. This is where the special vessel we call the “ductus arteriosus” comes into play.
This vessel acts as a bridge, a “shortcut” between the pulmonary artery, which carries blood from the right side of the heart to the lungs, and the aorta, the main artery that distributes clean blood throughout the body. This allows blood to bypass the lungs to a large extent and enter the body’s circulation directly. In short, the ductus arteriosus is present in every baby and is normal and essential for life in the womb. The real issue is not the presence of this vessel, but its failure to close at the expected time after birth. the word “patent” means “open” in Latin and summarizes the source of the problem.
When and why does the ductus arteriosus close?
When birth takes place and the baby takes its first breath, everything magically changes. The lungs fill with air and take over the task of oxygenating the blood. This triggers a series of radical changes in the circulatory system. The pulmonary vessels dilate and the blood pressure in this area drops. At the same time, as the placenta leaves the placenta, the level of hormone-like substances called “prostaglandins”, which keep the ductus arteriosus open, decreases rapidly in the blood.
The increase in oxygen levels in the blood and the decrease in prostaglandins create a strong signal for the muscles in the wall of the ductus arteriosus to contract. As a result of this contraction, the vessel contracts and is functionally closed, usually within the first 48-72 hours after birth. Over the next few weeks, the vessel completely transforms into a connective tissue remnant (“ligamentum arteriosum”) and completes its function. PDA is when this normal closure process is interrupted for some reason and the vessel remains open.
How does an open PDA strain the heart and lungs?
After birth, the blood pressure in the aorta, which pumps blood throughout the body, is significantly higher than the pressure in the pulmonary artery, which pumps blood to the now functional lungs. When the PDA remains open, this pressure difference causes blood to flow continuously from the aorta to the pulmonary artery, as if leaking from a high-pressure pipe into a low-pressure pipe. In medicine, we call this a “left-to-right shunt” or leakage.
This abnormal blood flow causes two main problems. First, much more blood goes to the lungs than normal. The lungs have to cope with this unexpected and excessive volume of blood. This increases the pressure in the pulmonary arteries and over time can lead to “blistering” of the lungs (pulmonary edema), making breathing difficult.
Second, the workload of the heart increases. The excess blood that escapes into the lungs returns to the left side of the heart after passing through the lungs. This means that the left side of the heart has to pump a larger volume of blood with each beat than normal. Over time, this constant extra work can cause the heart to fatigue, the left chambers to enlarge and the heart muscle to weaken. How serious this effect is depends entirely on the diameter of the vessel that remains open, i.e. the size of the PDA.
What causes PDA and who is more at risk?
In most cases, there is no definite reason why the PDA does not close. However, it is well known that certain conditions increase the risk of PDA. Knowing these risk factors can help you understand the situation better.
The most important risk factors are the following:
- Premature (early) birth
- Genetic syndromes
- Family history of congenital heart disease
- Infections during pregnancy
When analyzed in more detail, prematurity is by far the most important risk factor. The earlier the gestational week of birth, the greater the likelihood of PDA. For example, more than half of babies born before 28 weeks have PDA. This is because the ductus tissue of premature babies is less sensitive to signals that trigger closure (such as increased oxygen) and their closure mechanisms are not yet fully mature.
Some genetic conditions may also be more common with PDA. The most well-known are:
- Down syndrome (Trisomy 21)
- Char syndrome
- Holt-Oram syndrome
- Noonan syndrome
Some infections that the mother has early in pregnancy can also increase the risk. In particular, rubella infection can significantly increase the risk of PDA by having adverse effects on the baby’s heart development. It is therefore important for women planning a pregnancy to review their vaccination schedule. Some other risk factors are as follows:
- Gender (about twice as common in female babies compared to male babies)
- Childbirth at high altitude (due to the low level of oxygen in the air at very high altitudes above sea level)
What are the most common symptoms of patent ductus arteriosus?
The symptoms of PDA vary greatly depending on the severity of the condition, i.e. the size of the open vessel and the age of the patient. The clinical picture can range from a “silent” condition with no symptoms to life-threatening heart failure.
In small PDAs, the condition is usually completely asymptomatic. The growth and development of these babies and children is completely normal. The only symptom may be a continuous murmur during both contraction and relaxation of the heart, called a “machinery murmur”, which the doctor hears with a stethoscope during a routine examination. Some small PDAs may even go unnoticed until adulthood.
Moderate and large PDAs, on the other hand, cause significant symptoms, especially in infants. These symptoms are often a reflection of heart failure. As a parent, there are some important tips you should pay attention to:
Symptoms that can be seen in infants and young children include
- Rapid breathing
- Shortness of breath (especially during feeding or crying)
- Getting tired quickly while feeding
- Frequent cessation of sucking
- Sweating on the forehead or head during feeding
- Inadequate weight gain and growth retardation
- Faster heartbeat than normal (tachycardia)
- A general state of fatigue and restlessness
- Frequent recurrent lung infections (bronchitis, pneumonia)
In premature babies, the effects of a large PDA can occur earlier and more severely because their body systems are more sensitive. In addition to the above symptoms, these babies may develop more serious problems such as increased need for a respirator or sudden episodes of apnea.
People who live with a large PDA that is not diagnosed until adulthood may develop symptoms over the years due to fatigue of the heart and lungs. These symptoms may include:
- Shortness of breath that increases with exertion (such as walking, climbing stairs)
- Rapid fatigue and weakness
- Heart palpitations
- Rhythm disorders (arrhythmia)
How do doctors confirm the diagnosis of PDA?
The diagnosis of PDA usually starts with a careful physical examination, followed by simple, painless tests. The process usually involves the following steps:
Physical Examination: It usually starts with the doctor’s stethoscope. The strongest suspicion for a PDA is when a continuous murmur is heard, such as the typical “machine murmur” or “train noise” mentioned above. Also, with large PDAs, the doctor may notice that the pulses are fuller and more jumpy than normal.
Echocardiography (ECHO): This test is considered the “gold standard” for diagnosing PDA. It is an ultrasound of the heart, a painless and harmless method that uses sound waves to visualize the structure of the heart, its chambers and blood flow in real time. ECHO directly visualizes the open ductus between the aorta and the pulmonary artery, measures its diameter and clearly assesses how much blood is leaking through this opening (the amount of shunt). Most importantly, the ECHO shows whether this leakage leads to an enlargement of the left side of the heart. This finding plays a key role in deciding whether PDA requires treatment.
Other Ancillary Tests:
Chest X-ray (chest X-ray): In large PDAs, due to increased blood flow to the lungs, the pulmonary vessels may be prominent and the shadow of the heart may be enlarged (cardiomegaly). In small PDAs, it is usually completely normal.
Electrocardiogram (ECG): This simple test, which transcribes the heart’s electrical activity on paper, may provide indirect evidence that the left side of the heart overworks in large PDAs.
As a result of these tests, the diagnosis of PDA is confirmed and a treatment plan is created according to its size and its effect on the heart.
What are the long-term consequences of an untreated PDA?
When deciding on treatment, it is very important to consider the potential long-term risks of PDA. These risks largely depend on the size of the PDA. A small PDA that does not burden the heart may not need urgent closure. But waiting on a large PDA can increase the risk of irreversible damage.
The most serious complications of untreated medium and large PDAs include
- Pulmonary Hypertension (Lung Hypertension)
- Eisenmenger Syndrome
- Congestive Heart Failure
- Infective Endocarditis (Heart Infection)
- Growth and Development Retardation
The most serious of these complications is Pulmonary Hypertension and its advanced stage, Eisenmenger Syndrome. The constant pumping of high-pressure blood into the pulmonary vessels causes the walls of these vessels to thicken, harden and narrow over time. This permanently increases the blood pressure in the lungs. As the disease progresses, the pressure in the lungs increases so much that the direction of blood flow reverses. This means that instead of clean blood escaping into the lungs, dirty blood from the lungs enters the body through the PDA. This leads to bruising (cyanosis) and is irreversible lung damage. After reaching this stage, it is no longer possible to turn off the PDA.
Congestive Heart Failure is a condition in which the heart becomes tired from constantly overworking and is unable to pump enough blood effectively to meet the body’s needs. It is characterized by symptoms such as feeding difficulties and shortness of breath in infants and fatigue and swelling of the legs in adults.
What are the current patent ductus arteriosus treatment options?
The method of PDA treatment is individually determined according to the patient’s age, weight, general health and, most importantly, the size and anatomy of the PDA. There are currently four main treatment approaches:
Observation and Waiting: A “wait and see” approach can be adopted especially for very small PDAs detected in the first months of life, which are asymptomatic and do not put any burden on the heart on ECHO. Because some of these can close by themselves.
Medication Therapy: This treatment is only effective in preterm (premature) babies, usually in the first days of life. It does not work in full-term babies, children or adults. Drugs such as Indomethacin or Ibuprofen, given intravenously, can help the duct to contract and close.
Angioplasty (Transcatheter) Closure: Today, it is the first-choice, standard treatment method for most pediatric and adult patients with appropriate anatomy. It is a non-surgical, minimally invasive procedure.
Surgical Closure Surgery is the gold standard treatment in cases where drug therapy fails, angioplasty is not technically feasible (e.g. very small premature babies or anatomically inappropriate PDAs) and urgent intervention is required.
How is PDA closure performed by angiography, a non-surgical method?
Transcatheter PDA closure is not performed in an operating room but in a specially equipped angiography (catheter) laboratory. The procedure is usually performed under general anesthesia, so the patient (especially children) does not feel anything during the procedure.
The procedure steps are simple. A small needle is inserted into a large vein (femoral vein), usually in the groin area. A thin sheath is placed over this access site, which allows the catheter to move freely through the vein. The catheter, a long and flexible tube, is guided through this sheath to the heart and then to the pulmonary artery under the guidance of X-ray imaging.
To clarify the exact location, size and shape of the PDA, an angiogram is performed with contrast medium (dye). Based on these measurements, the selected closure device (such as an umbrella, plug or coil) is advanced through the catheter via a long guidewire. The device is placed like a plug just inside the PDA, between the aorta and the pulmonary artery. After checking that the device is in the correct position and has completely stopped blood leakage, it is detached from the delivery system and left permanently inside the PDA.
The main advantages of this method are that the breast does not need to be opened, there are no surgical scars, the hospital stay is very short (usually one night) and the return to normal life is much faster.
When and how is PDA surgery performed?
Surgical treatment is the best and sometimes the only option in certain situations where medication is ineffective or angiography is not appropriate. It is especially life-saving in very small premature babies who cannot be weaned from the ventilator or who show signs of heart failure. Surgical closure is also preferred for large PDAs whose anatomical structure (e.g. too wide, short or calcified) is not suitable for the safe placement of angiographic devices.
PDA surgery is a “closed heart surgery” that does not go inside the heart and usually does not require a heart-lung machine. There are two main modern surgical approaches:
Traditional Open Surgery (Thoracotomy): This is the standard procedure that has been used safely for many years. The operation is performed under general anesthesia. The patient is placed on their side and a small incision is made under the left shoulder blade, between the ribs. Through this incision, the chest cavity is entered, the lung is gently moved aside and the PDA vessel is clearly visible. The surgeon either ties the vessel tightly with a strong suture material (ligation) or places a special titanium clip to close the vessel completely (clipping).
Minimally Invasive Surgery (VATS): This is a modern and less traumatic alternative to traditional open surgery. Instead of a large surgical incision, this technique uses a camera and specialized surgical instruments through 2-3 very small (3-5 mm) holes in the chest wall. The surgery is monitored on a high-resolution screen. The camera provides a magnified and clear view of the surgical field, allowing very precise work on the PDA and adjacent important structures. The most important advantages of VATS include much less postoperative pain, a shorter hospital stay, a faster recovery and a much better cosmetic result:
What is the recovery and long-term follow-up process after treatment?
The recovery process varies depending on the treatment method applied. instead of thinking “I have been treated, it is over”, the approach should be “the problem is solved, now let’s protect our health”.
After closure by angioplasty: Recovery is extremely fast. Patients are usually discharged within 24 hours after the procedure. Children can return to their normal daily activities and play within a few days. It is only desirable to avoid heavy exercise or strenuous activities for a few weeks.
After Surgical Closure: After surgery, the patient, especially small babies, needs to stay in the hospital for a few days. Painkillers are given to control postoperative pain. Full recovery and full return to normal activities usually takes several weeks.
After both methods, there is a significant relief, especially in babies who previously had feeding difficulties. Because their breathing is relaxed and their heart is less tired, they are more energetic, eat better and their weight gain accelerates.
The long-term prognosis after a successfully closed isolated PDA is excellent. The vast majority of patients lead a completely normal, healthy and active life without any restrictions. It is standard practice to perform a cardiology check-up and ECHO within the first year after the procedure (usually at 1, 6 and 12 months) to confirm the success of the treatment. These checks confirm that the closure device is in place, that complete closure has been achieved and that the size of the heart chambers has returned to normal. After a successful and uncomplicated closure procedure, a lifetime of strict cardiologic follow-up is usually not necessary and a healthy, active and fulfilling future awaits you or your child.

Prof. Dr. Yavuz Beşoğul graduated from Erciyes University Faculty of Medicine in 1989 and completed his specialization in Cardiovascular Surgery in 1996. Between 1997 and 2012, he served at Eskişehir Osmangazi University Faculty of Medicine as Assistant Professor, Associate Professor, and Professor, respectively. Prof. Dr. Beşoğul, one of the pioneers of minimally invasive cardiovascular surgery in Türkiye, has specialized in closed-heart surgeries, underarm heart valve surgery, beating-heart bypass, and peripheral vascular surgery. He worked at Florence Nightingale Kızıltoprak Hospital between 2012–2014, Medicana Çamlıca Hospital between 2014–2017, and İstinye University (Medical Park) Hospital between 2017–2023. With over 100 publications and one book chapter, Prof. Dr. Beşoğul has contributed significantly to the medical literature and is known for his minimally invasive approaches that prioritize patient safety and rapid recovery.